Structural Representations of Organic Compounds
Structural Representations of Organic Compounds: Overview
This topic covers concepts, such as, Structural Representation of Organic Compounds, Complete Structural Formulas of Organic Compounds, Wedge-and-dash representation of CH4 & Molecular Models of CH4 etc.
Important Questions on Structural Representations of Organic Compounds
The number of and bonds present in the given compound, respectively, are


The number of and bonds in the compound
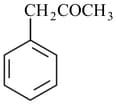 respectively are
respectively are
What is the correct formula of neopentane?
Draw the complete formula of methane according to the structural model.
In Wedge-Dash notation for three-Dimensional representation of an organic molecule, the dashed bond represents.
_____ model only the bonds connecting the atoms of a molecule and not the atoms themselves are shown in the molecule of methane.
In the ball-and-stick model, both the atoms and the _____ are shown in the molecule of methane.
The space-filling model of methane emphasises the relative size of each atom based on its van der Waals radius.
A compound contains and only, and has molecular mass Its photochlorination gives a mixture containing only one monochloro and two dichloro hydrocarbons. Deduce the structure of
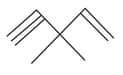
How many pi bonds and sigma bonds are present in following molecule?
Newman projection of staggered conformation of ethane is
Give the structure of the following compound:
-Trichloroethane
Give the structure of the following compound:
p-Dichlorobenzene
Give the structure of the following compound:
-Chlorobutane
Give the structure of the following compound:
-Bromo--ethyl--methylhexane
Define framework model of ?
Define space filling model of .
Wedge-and-dash representation is used to show non-planar bonds in the molecule.
hybridised molecule with tetrahedral geometry of bonds( Wedge-and-dash representation of ).
Draw the wedge-and-dash representation of .
